Our Science
- Home
- Our Science
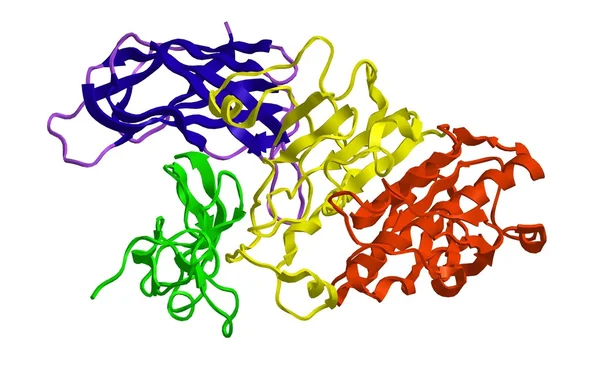
MYC proteins play a key role in up to 70% of all human cancers and are implicated in tumorigenesis and therapeutic resistance mechanisms. Specifically, the heterodimer of MYC and related transcription factor MAX regulates several downstream genes primarily involved in the cell cycle. Silencing or inhibition of MYC thus results in resensitization of tumors to extant therapies. Despite the enormous promise of MYC- targeting cancer treatments, there exist no direct small-molecule MYC inhibitors in the
clinic.
Several conceptual and practical difficulties, including MYC’s lack of defined binding “pockets” and potential toxicity to normal tissues have led many to regard MYC as an “undruggable” target. Nevertheless, our team is leveraging evolving computational strategies with unique insights into MYC protein binding and function, in combination with in vivo screening to rapidly identify and evaluate potential inhibitors.
Small-Molecule MYC Inhibitors Suppress Tumor Growth and Enhance Immunotherapy
Summary
A MYC inhibitor selectively alters the MYC and MAX cistromes and modulates the epigenomic landscape to regulate target gene expression
Introduction
14-3-3 Proof of Concept and Possibilities
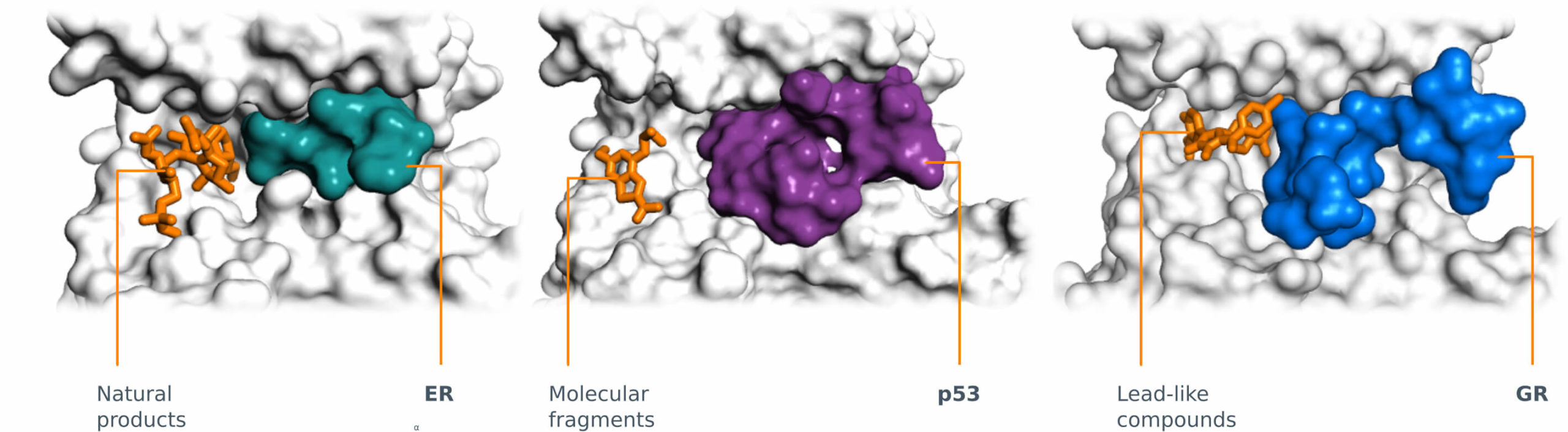
14-3-3 modularity
Selected Publications
14-3-3 and Carbohydrate Response Element Binding Protein (ChREBP)
Nature Communications, August 7, 2020
Site-directed fragment-based screening for the discovery of protein–protein interaction stabilizers
14-3-3 and Estrogen Receptor alpha (ERα)
American Chemical Society, February 1, 2019
Fragment-based differential targeting of PPI stabilizer interfaces
14-3-3 and p53 or TAZ
American Chemical Society, June 5, 2020
14-3-3, ERα and Fusicoccin
PNAS, May 15, 2013
American Chemical Society, March 16, 2023
Nature Communications, June 23, 2022
